Qorvo® (Nasdaq: QRVO), a leading global provider of connectivity and power solutions, today announced that the U.S. Food and Drug Administration (FDA) has granted emergency use authorization (EUA) for the Qorvo Omnia™ SARS-CoV-2 Antigen Test in Point-of-Care (POC) settings.
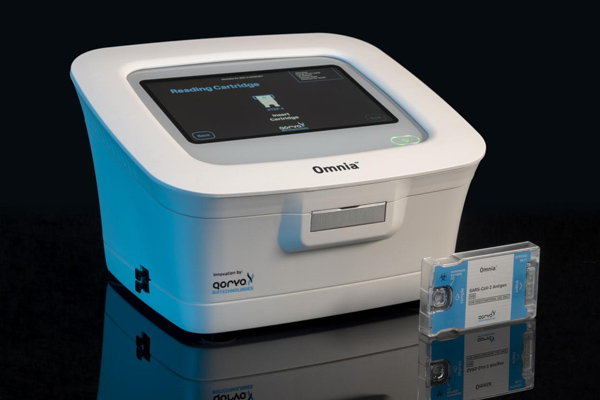
The test is authorized for the qualitative detection of nucleocapsid viral antigens from SARS-CoV-2 (COVID-19) in nasal swab specimens from individuals who are suspected of COVID-19 within six days of symptom onset. The test is also authorized for individuals without symptoms or other epidemiological reasons to suspect COVID-19 when tested twice over three days, with at least 24 hours and no more than 48 hours between tests. The FDA previously granted an EUA for use in moderate and high complexity settings such as laboratories. This EUA significantly expands the market for Qorvo beyond laboratories to include physician offices, urgent care centers, retail pharmacies, employee health testing, and any other locations where CLIA waived tests can be performed.
Erik Allen, recently appointed vice president of Qorvo and president of Qorvo Biotechnologies, said, "As the COVID testing market transitions to an endemic state and the increase in the omicron variant drives lower viral load, high quality rapid testing infrastructure is needed in POC settings. The Qorvo Omnia platform provides a unique combination of performance, automated workflow and scalability to serve on-site testing needs in a very efficient manner."
The Qorvo Omnia platform utilizes an innovative diagnostic technique by using high frequency Bulk Acoustic Wave (BAW) sensors to achieve highly sensitive and specific COVID-19 detection results with a fast turnaround time, in an easy to use, self-contained platform. BAW sensor technology enables low limit of detection (LOD) levels similar to molecular testing capability. As viral loads decrease particularly with the Omicron variant and sub-variants, low viral load in patient samples presents challenges for existing over-the-counter (OTC) lateral flow antigen tests, giving the Qorvo Omnia platform a technological advantage to continue to accurately detect antigen associated with COVID-19 during Omicron surges.
The Qorvo Omnia platform demonstrates excellent performance at the LOD (Limit of Detection) for the comparator PCR method. The Omnia platform demonstrated 85% sensitivity (PPA) during clinical studies to support the device Emergency Use Authorization at the Point of Care (POC); this NIH-sponsored study took place in the latter part of 2021. A separate NIH-sponsored research study was conducted during Omicron variant circulation at the beginning of 2022. During this study, Qorvo continued to see exemplary performance at 86% sensitivity where samples were at or above the LoD of the comparator PCR method. In all studies, 100% specificity was observed.
Peter Matos, president and CEO of medical consultancy Traeokos, said, ¡°Qorvo's antigen test brings the level of performance my clients need in multiple testing scenarios - especially as testing needs have evolved with Omicron variant performance challenges.¡±
For more information, visit www.qorvobiotech.com.
This project has been funded in whole or in part with Federal funds from the National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health, Department of Health and Human Services, under Contract No 75N92021C00008.
The Qorvo Omnia SARS-CoV-2 Antigen Test was granted emergency use authorization (EUA) from the U.S. Food and Drug Administration (FDA) in April 2021 for use in moderate and high complexity settings and as of July 2022 for Point of Care settings.
The Qorvo Omnia SARS-CoV-2 Antigen Test has not been FDA cleared or approved. It has been authorized by the FDA under an Emergency Use Authorization and testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. ¡ì263a, to perform waived, moderate or high complexity tests. This test has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens. These tests are only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostic tests for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. ¡ì 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
About Qorvo Biotechnologies
Qorvo Biotechnologies, LLC is a wholly owned subsidiary of Qorvo, Inc. focused on the development of point-of-care (POC) diagnostics solutions leveraging Qorvo's innovative BAW sensor technology.